The uterus is essential for reproduction in most mammalian species and is arguably the most naturally plastic organ in terms of tissue remodeling in mammals. Histologically, the uterus is divided into the endometrium and the outer smooth muscle layer called the myometrium. The endometrium harbors epithelial (glandular and luminal) and fibroblast-like stromal cells, and is separated physiologically into the functionalis (inner most region nearest the lumen) and basalis layers. The key morphological changes that the uterus undergoes are in response to cyclical hormonal cues from the ovary and from the implanting embryo during pregnancy. During menses and following parturition, the functionalis layer of the endometrium regresses and is lost in primates and must be replaced. During pregnancy, the wet weight of the uterus increases 10-fold largely due to myometrial smooth muscle cell hypertrophy and hyperplasia. We believe that the remodeling of the uterus in response to these stimuli and its return to a basal state requires adult stem (or progenitor) cells that reside in the individual endometrial and myometrial compartments. Furthermore, we suspect that several pathological conditions, such as endometrial cancer, endometriosis, and leiomyomata (i.e., uterine fibroids), can be attributed to dysregulation of these same stem cells, or are derived from committed cells that acquire stem-like features. We will review uterine development and its response to hormonal cycling and pregnancy, uterine neoplasia, and the evidence for a role for uterine stem cells in these settings.
1. Introduction
Nearly all species of mammals are viviparous; that is, they give birth to live offspring, which has been an extremely successful evolutionary strategy for mammalian species. The increased energy demands associated with rearing offspring for a longer period of time replaces the need for increased energy expenditure to produce larger numbers of offspring for maintenance of the species, such as occurs with invertebrates or lower vertebrates (e.g., amphibians). In most mammals (i.e., eutherian), giving birth to live offspring is made possible because the uterus performs placental-like functions until the embryo can develop its own placenta for nutrient and gas exchange from the mother (or biological host).
The uterus (see Figure 1A) is by far the largest female reproductive tract organ, the primary function of which is to nurture the implanted embryo/fetus throughout gestation. The uterus is a remarkably resilient organ that undergoes two postnatal developmental programs under the direction of ovarian-derived steroid hormones. The first occurs in a cyclical fashion in the absence of conception and is referred to as the menstrual cycle in humans and other primates, and the estrous cycle in non-primate species. Estrogen levels are normally elevated during the proliferative phase of the menstrual/estrous cycle and serve to promote proliferation of the luminal and glandular epithelial cells associated with the thickening of the endometrial lining. This in turn gives rise to the progesterone dominant secretory phase. In primates the endometrium can be compartmentalized functionally into the more superficial functionalis and the underlying basalis (see Figure 1B). The continuous cyclic nature of the endometrium through the female's reproductive lifetime implies that regeneration of the glandular and stromal components is likely a product of a stem and/or a progenitor (transient amplifying) cell population. Although more differentiated than stem cells, the progenitor/transient amplifying cells retain limited ability to undergo symmetric division generating more differentiated daughter cells. This uterine cycle involves sequential periods of cellular proliferation, differentiation and regression. In the absence of embryonic implantation, the end result of this cycling is menstruation where the endometrial mucosal lining nearest the luminal compartment, the functionalis layer (shown in Figure 1B), which includes the apical portion of the endometrial stroma, is shed. The uterus then prepares for another round of proliferation and tissue remodeling in the event that fertilization should ensue (Reviewed in Jabbour et al., 2006). In rodents, the epithelium undergoes similar cycles of proliferation in response to ovarian steroid hormones. In contrast, the effects of ovarian steroid hormones and estrous cyclicity on the stroma and myometrium have not been as well studied.

The female reproductive tract is shown in panel A comprising a cervix, uterus, and Fallopian tubes. Histologically, the uterine wall can be subdivided into the outer perimetrium, inner endometrium, and intermediate myometrium. Panel B shows cyclic changes during the menstrual cycle. In the sexually mature woman, the uterus goes through monthly cycles in which the functionalis portion of the endometrial lining of the uterus is stimulated to grow by estrogen, which is produced by the ovary. After ovulation, progesterone is also produced by the ovary causing the lining of the uterus to stop growing. If pregnancy is not established, the functionalis is mostly shed, i.e. menstruation.
The second developmental process occurs during pregnancy when maternal stromal cells adjacent to the uterine lumen terminally differentiate, a process referred to as decidualization. Uterine decidualization is required for the establishment and maintenance of pregnancy in invasively implanting (i.e., hemochorial) species such as humans and rodents. In humans the wet weight of the non-gravid uterus is approximately 100 g but at term it can weigh 10 times that and returns to non-gravid weight by 4–5 weeks postpartum (Morrione and Seifter, 1962). Similarly, the mouse uterus can also expand by 10-fold in weight to support the development of multiple offspring with a ‘beads on a necklace’ morphology (Rosendaal, 1975). These remarkable changes are accomplished initially by increased hypertrophy and hyperplasia, and are then followed by apoptosis and necrosis after parturition. Based on the dynamic tissue remodeling in all compartments of the uterus, both during the menstrual/estrous cycle and pregnancy, it has been suggested that adult stem cells must play a prominent role in uterine tissue maintenance and function. Here we provide a brief overview of the data supporting the existence of uterine adult stem cells and discuss the role these cells likely play during normal adult uterine physiology. We also consider evidence for the contribution of uterine stem cells, when gone awry, to uterine disease (e.g., fibroids and cancer).
2. Uterine development
2.1. Müllerian duct formation and neonatal development
The internal female reproductive tract structures in vertebrates are derived from the embryonic Müllerian ducts (alternatively known as the paramesonephric ducts), which are formed by invagination of coelomic epithelial stoma followed by cranial to caudal tubulogenesis along the existing Wolffian ducts (alternatively known as the mesonephric ducts; Jacob et al., 1999) in the bipotential or sexually indifferent urogenital ridges of the intermediate mesoderm found along the posterior wall of the abdominal cavity (reviewed in Kobayashi and Behringer, 2003). The extension of the Müllerian duct epithelium occurs through proliferation of the cells at the leading end of the duct (see Figure 2; Orvis and Behringer, 2007).
A. Before the embryonic to fetal transition, the gonads and reproductive ducts (Wolffian; blue and Müllerian; red) of the urogenital ridge are bipotential. B. In the presence of the Y chromosome, the gonads of the bipotential urogenital ridge differentiate into testes, which produce both MIS to eliminate the Müllerian ducts and testosterone to stimulate differentiation of the Wolffian ducts into the male internal reproductive tract structures. C. In the absence of SRY, ovaries differentiate, Wolffian ducts degenerate, and Müllerian ducts develop into a simple columnar epithelial tube that will differentiate into the oviducts, uterus, cervix, and upper portion of the vagina.
The bilateral Müllerian ducts cross the Wolffian ducts ventrally and continue to grow caudomedially where they become adjoined at the midline and fuse to form the uterine canal (Fig. 2). The degree of fusion below the oviducts varies between species. Whereas there is limited fusion in most mammalian species, which generally results in uteri with two horns, most primates have near complete anterior fusion to form a simplex uterus.
In mammals, the sexually dimorphic expression of SRY (Sex-determining region of the Y chromosome) in the differentiating male testes triggers a transcriptional response that, among other events, results in the expression of Müllerian Inhibiting Substance (MIS, also known as antiMüllerian hormone or AMH), which is required for Müllerian duct regression in males (reviewed in (Josso et al., 2006; Teixeira et al., 2001)). In the absence of MIS or its single transmembrane serine/threonine kinase receptors results in a rare form of pseudohermaphroditism known as Persistent Müllerian Duct Syndrome in males (Belville et al., 1999). In females, absence of SRY and the subsequent lack of MIS expression in the developing ovaries result in retention of the Müllerian ducts and their differentiation into the internal female reproductive tract structures.
Despite the relative importance of the Müllerian duct-derived uterus for reproduction, little is known about the molecular mechanisms that regulate their embryonic or postnatal phases of differentiation (for a comparative review see Spencer et al., 2005). Some of the genes needed for early events in Müllerian duct formation have been determined by mutagenesis studies in mice. Müllerian duct agenesis has been described in mutants lacking the homeodomain transcription factors PAX2 (Torres et al., 1995), LIM1 (Kobayashi et al., 2004), and Emx2 (Miyamoto et al., 1997) as well as in mice lacking wingless-type MMTV 4 (Wnt4; Vainio et al., 1999) and Wnt9b (Carroll et al., 2005), members of the WNT gene family of secreted signaling molecules (reviewed in Kobayashi and Behringer, 2003). Another WNT family member, Wnt7a, is expressed in the luminal epithelium of the relatively simple uterine tube and is necessary for controlling postnatal differentiation along the anterior-posterior and radial axes of the epithelial and stromal layers (Miller and Sassoon, 1998; Parr and McMahon, 1998). Wnt5a, a third member of the WNT family expressed in the uterine stroma (Mericskay et al., 2004), along with Wnt7a, and the spatial co-linear expression pattern of homeobox proteins, Hoxa9, Hoxa10, Hoxa11, and Hoxa13 (Taylor et al., 1997; Benson et al., 1996; Branford et al., 2000; Post and Innis, 1999), are also necessary for the differential morphogenesis of the cervix, oviducts and uterine corpus from the primitive uterine tube (see Figure 2).
2.2. Postnatal development
In rodents, the early postnatal mesenchyme of the primitive uterine tube differentiates into two of the adult layers of the uterus: the endometrial stroma and the myometrial muscle layers (Kurita et al., 2001; see Figure 3). Perinatally, the mouse uterus contains a luminal endometrial layer consisting of a single columnar epithelium supported by a stromal layer that has no endometrial glands and the circular and longitudinal smooth muscle layers of the myometrium become discernable (Brody and Cunha, 1989). By postnatal day 15, the myometrium is well developed and the endometrial glands are visibly coiled and comparable to those observed in the adult uterus. As with stromal and epithelial differentiation, there is also evidence of Wnt participation in adenogenesis (Mericskay et al., 2004; Miller and Sassoon, 1998; Parr and McMahon, 1998). In humans, the uterine architecture at birth resembles that of the adult, though less well developed, and the glandular epithelium is derived initially from projections of the luminal epithelium (reviewed in Gray et al., 2001).
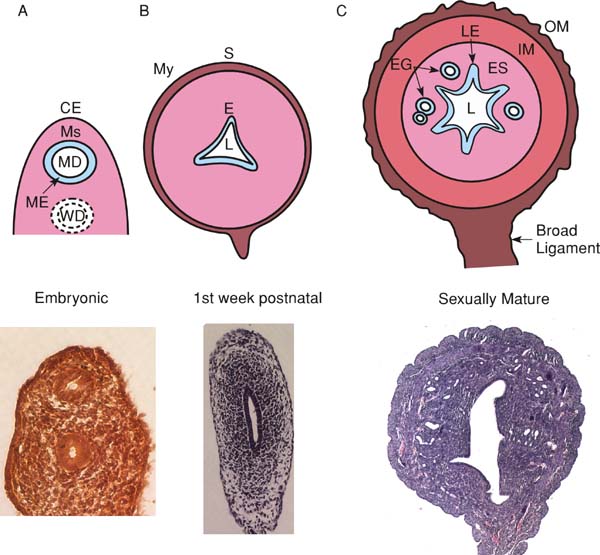
A. The fetal uterus is a simple tube with mesenchyme (Ms) between the inner Müllerian duct (MD) and outer coelomic epithelia (CE). B. By postnatal day 3, the endometrium (E) consists of a single columnar epithelial layer supported by stroma (shaded), the myometrial layers (My) are discernable and enclosed within the serosa (S), and the lumen (L) is slightly folded. C. After day 15, the endometrial glands have formed and the uterus is histologically similar to that of an adult.
Correct differentiation of the uterine epithelium requires unknown signals from the uterine mesenchyme but appears to be independent of either classical estrogen receptors (ER) or progesterone receptors (PR), since uterine development is essentially normal in ER knockout (Lubahn et al., 1993) and PR knockout mice (Lydon et al., 1995). Conversely, recent studies suggest that WNT signaling is necessary for mediating the actions of estrogen in the uterus (Ray et al., 2008). The molecular details involved in the development of the radial axis of the simple perinatal uterine tube and its differentiation into the mature uterus have not been described. Additionally, although the ovarian-derived steroid hormones estradiol and progesterone have been known for some time to regulate the uterine cyclicity, still largely unknown are the signaling mechanisms required for regeneration of the adult uterine endometrium and remodeling of the myometrium at the end of each cycle and following pregnancy.
3. Adult uterine stem cells
3.1. Endometrial stem cells
Recent advances in stem cell biology have made it clear that most tissues are extremely plastic, exhibiting renewal through adult stem or progenitor cells. Yet, only a handful of studies consider adult somatic stem cells in female or male reproductive organs. On the whole, studies of adult stem cell biology in the uterus lag far behind other areas of stem cell research despite the fact that, compared with other organs, the uterus undergoes perhaps the most extensive proliferative changes and remodeling in adult mammals.
Some studies of endometrial stem cells have focused on the existence of an extrauterine source of adult stem cells that are presumably recruited to the uterus with each menstrual/estrous cycle, and that it is these cells that are responsible for repair of the uterus following menses or parturition. One proposed source could be bone marrow-derived mesenchymal stem cells. Bone marrow transplantation into radiation- or chemotherapy-conditioned recipient mice has revealed that bone marrow harbors cells that contribute to the tissue architecture of a number of organs at relatively low levels (Bruscia et al., 2006). Independent data generated from two laboratories suggests a role for bone marrow contributions to the uterine endometrium. In a human study, uterine tissues were obtained from four women following hysterectomy who previously had received a single bone marrow transplant following chemotherapeutic conditioning. Because HLA antigens were different between donor marrow and recipients, donor-derived cell could be tracked in endometrial tissues by PCR and immunohistochemistry. Using both methods between 2% and 52% of the recipients’ endometrial tissue was HLA-mismatched, or donor-derived. Interestingly, donor-derived cells were found in both the epithelial and stromal compartments of the endometrium (Taylor, 2004). In a subsequent study completed by the same laboratory, female mice were conditioned for transplantation of 1 × 107 unfractionated male bone marrow cells by lethal irradiation (Du and Taylor, 2007). Y-chromosome fluorescence in situ hybridization was used to detect male bone marrow-derived cells in both the stromal and epithelial compartments. While no clonal expansion was observed as expected if bone marrow contributed cells with stem cell qualities to the uterus, individual Y-chromosome positive, CD45 negative cells were observed in the epithelial (0.02%) and stromal (0.03%) compartments. These bone marrow transplantation studies were recently corroborated using GFP transgenic mice (Bratincsak et al., 2007). While it is clear that intravenous transplantation of bone marrow cells does result in the presence of a small number of cells in endometria of conditioned (i.e., lethally irradiated) recipients, and the therapeutic potential for these findings are of great importance, three issues must now be resolved. First, are these donor-derived cells functional within the endometrium by contributing to physiological processes such as pregnancy or endometrial repair during the normal estrous/menstrual cycle? Second, do the donor-derived cells possess stem cell qualities? Third, does the endometrium recruit bone marrow cells under physiological conditions? These important questions are not restricted to studies of endometrial adult stem cells; in fact, they are a major driving force in adult stem cell biology across diverse tissues and organ systems. Experimental data supporting the existence of endogenous endometrial stem/progenitor cells has come recently from several correlative experiments utilizing different methods.
First, clonogenicity, as defined by the ability of single cells to proliferate and produce isolated colonies of progeny when seeded in culture at very low density, is a classical approach used as an initial step to test the presence of stem or progenitor cells in a given tissue. Chan et al, demonstrated that the human endometrium harbors clonogenic epithelial and stromal cell populations (Chan et al., 2004). Approximately, 0.15% of epithelial and 1.3% of the stromal cell populations are clonogenic (Chan et al., 2004; Schwab et al., 2005). Interestingly clonogenicity within the stromal and epithelial cell fractions do not differ between actively and inactively menstruating women (Schwab et al., 2005), suggesting that ovarian-derived steroid hormones do not maintain the clonogenic potential of uterine epithelial and stromal tissues. Based on in vitro studies, growth factors supporting clonogenic cells include epidermal growth factor (EGF), platelet-derived growth factor BB (PDGF-BB), and transforming growth factor (TGF) α (Schwab et al., 2005). Clonogenicity studies of human stromal stem/progenitor cells have been bolstered by in vitro differentiation assays. For instance, unfractionated human endometrial stromal cell cultures can be induced to follow a chondrogenic differentiation pathway, suggesting the presence of mesenchymal stem cells in the endometrium similar to those found in bone marrow (Wolff et al., 2007). This finding is supported by more extensive differentiation studies in which rare (1.5%) CD146+/PDGF-Rβ+ stromal cells could be induced to differentiate into osteocytes, chondrocytes, myocytes, and adipocytes (Schwab and Gargett, 2007; Schwab et al., 2008). Immunohistochemical studies revealed that these highly clonogenic and multipotential cells reside near the vasculature (Schwab and Gargett, 2007). Studies of other organ systems suggest that endogenous tissue-committed stem/progenitor cells maintain tissue repair and homeostasis. The idea that the endometrium contains stem or progenitor cells has been proposed several times over the past three decades (Padykula et al., 1989; Prianishnikov, 1978; Tanaka et al., 2003). In primates, endometrial epithelial stem/progenitor cells are believed to be located within a niche deep in the basalis region near the interface of the stroma and myometrium. This subpopulation is thought to be relatively unresponsive to the differentiating effects of steroid hormones (Padykula, 1991) and serves as the feeder source for the clonally-derived epithelial glands in the immediate and adjacent areas (Tanaka et al., 2003). Perhaps the best example of clonality in vivo comes from studies of the human endometrium where immunohistochemistry was used to identify PTEN mutant glands (i.e., those deficient in PTEN protein) in remnants of the endometrial lining following menses. PTEN negative cells gave rise to PTEN negative glandular structures in the immediate area in ensuing proliferative phases (Mutter et al., 2001).
A second method used to identify putative stem/progenitor cells is retention of DNA label. Here, mice are first given a pulse of a DNA labeling agent such as 5-bromo-2-deoxyuridine (BrdU) and then chased for several weeks to months with no treatment. Adult stem cells usually divide infrequently and thus retain BrdU for a longer period than differentiated cells. Due to dilution through cell replication, the BrdU label is eventually lost in the rapidly dividing cells while stem/progenitor cells retain label. Spatial orientation of such label retaining cells (LRCs) can then be determined in situ through histological sectioning. This approach has been used by two laboratories in efforts to identify LRCs (i.e., presumably stem/progenitor cells) in the murine endometrium (Cervello et al., 2007; Chan and Gargett, 2006). The BrdU label was retained long term in 6–9% of the stromal cells, the majority of which localized to the myometrial:stromal interface with approximately a third being found in the subluminal stromal compartment (Cervello et al., 2007; Chan and Gargett, 2006). Within epithelial tissue, LRCs were more rare accounting for about 3% of the total population by eight weeks into the chase phase of the experiment (Chan and Gargett, 2006). While all of the epithelial LRCs and most of the stromal LRCs were estrogen receptor (ER) negative, 16% of the stromal LRCs were found to express ER. This minor population of endometrial stromal cells may represent a unique population of estrogen responsive stem/progenitor cells. Interestingly, while most epithelial and stromal LRCs were ER negative, those localized to the myometrial:stromal interface could be stimulated to proliferate in response to estrogen treatment (Chan and Gargett, 2006). This observation suggests the indirect or intermediate involvement of committed stromal cells that respond to estrogen treatment by releasing stem/progenitor cell proliferating factors. Within the stromal compartment, a small percentage of the LRCs located at the basal aspect of the stromal compartment were shown to expresses c-kit (0.32%) or Oct-4 (0.19%). While the identity of these c-kit+ and Oct4+ LRC cells remains in question (i.e., whether they are endometrial stromal or immune cells) these studies at least provide further support for the existence of endogenous uterine stem/progenitor cells.
A third approach that has been used to study endometrial stem/progenitor cells is to characterize them on the basis of Hoechst 33342 dye exclusion through ATP-binding cassette transporters. Those cells capable of excluding Hoechst dye constitute the side-population (SP) during fluorescence-activated cell sorter (FACS) analysis. Kato et al. established that 0.00–5.11% of the cells in normal human endometrium constitute the SP fraction. While the clonogenic potential of these cells was not determined, they could be induced to differentiate in vitro into both epithelial- and stromal-like cells using long term Matrigel cultures (Kato et al., 2007).
With regard to endometrial epithelial stem/progenitor cells, critical experiments are still needed to determine exactly how the uterine epithelium is maintained. Based on the classical definition, a stem cell is one that has the capacity for self-renewal, based originally on serial transplantation experiments, and one that can differentiate into a variety of cell types. Bona fide epithelial stem cells with limited in vivo differentiation potential have been characterized in a number of adult tissues including skin (i.e., bulge cells), breast, intestinal, and prostate (Barclay et al., 2008; Cotsarelis et al., 1990; Loeffler et al., 1993; Shackleton et al., 2006). In contrast, other adult tissues such as pancreatic islets and renal tubules are maintained not by adult epithelial stem cells, but rather by self-replication of differentiated post-mitotic cells (Brennand et al., 2007; Humphreys et al., 2008). In the case of pancreatic β-cells, tissue homeostasis in the adult is maintained by: 1) differentiated cells that retain high proliferative capacity; or 2) committed cells that dedifferentiate in response to cues from surrounding cells followed by subsequent cell replication (Brennand et al., 2007). It appears that different epithelial tissues utilize different mechanisms for tissue repair and maintenance. Whether the endometrial epithelium is maintained by rare stem/progenitor cells, or if committed epithelial cells can be stimulated to dedifferentiate and then proliferate remains to be established.
3.2. Myometrial stem cells
The evidence for myometrial stem cells is much less abundant. This may be due, in part, to the dogmatic view that, outside pregnancy, the myometrium was a relatively quiescent tissue. However, uterine leiomyomas (or uterine fibroids) provide the clearest evidence suggesting adult stem cells in the human myometrium. These benign smooth muscle tumors are found in over 25% of adult women, are clonal in origin, and hormone-responsive. Patients may have a single uterine fibroid but many have multiple fibroids and they can be located anywhere in myometrial tissue. The size of the fibroids can vary greatly from microscopic to tumors weighing more than a kilogram and are so named because of the profuse collagen deposition by the smooth muscle cells. The clonality of fibroids argues for their development from a single dysregulated cell (Lobel et al., 2006; Townsend et al., 1970).
Mice do not normally develop fibroids but recent studies performed in one of our laboratories shows that conditional deletion of β-catenin in the Müllerian duct mesenchyme, results in a progressive loss of smooth muscle cells with their replacement by adipose cells in the myometrium (Arango et al., 2005). We then showed that there was a stem or progenitor cell in the myometrium that retained label in a pulse chase experiment and that was hormonally responsive (Szotek et al., 2007). Label-retention has been suggested as characteristic of stem or progenitor cell because of their relatively slower rate of division and the tendency of the preponderance of newly synthesized DNA segregating with the daughter strand, which fall under the rubric of the Immortal Strand Hypothesis (Cairns, 1975). After a long chase period, label-reatining cells (LRC) in the myometrium were often found adjacent to a dimmer label retaining cell that was also c-kit positive, suggesting a direct lineage for these cells (see Figure 4A). C-kit is the tyrosine kinase receptor for Stem Cell Factor and the proto-oncogene with frequent activating mutations in progenitor cell-derived cancers (Lennartsson and Ronnstrand, 2006). We also showed that there was an enrichment of Müllerian duct mesenchyme-derived cells by side population (SP) flow cytometry analysis (Szotek et al., 2007), a cell-sorting procedure that exploits the propensity of stem cells to efflux Hoechst dye because of a relative overexpression of ATP transporter or pump proteins (Goodell et al., 1996). Encouragingly, another report recently showed that SP cells isolated from human myometrium could differentiate into smooth muscle cells when injected into the mouse myometrium (Ono et al., 2007; see Figure 4B and C). Importantly, this group also showed that non-SP cells grafts did not express smooth muscle actin and that, in addition to their differentiation in vivo into smooth muscle cells, these human myometrial SP cells could be induced to differentiate in both osteogenic and adipogenic pathways in vitro. These results strongly suggest that a resident population of myometrial cells can functionally behave like myometrial stem cells. Many questions remain to be answered. What is the identity of the LRC/SP cells? What is their niche environment, if any? Are uterine fibroids the result of dysregulated function of these myometrial LRC/SP stem/progenitor cells?

Panel A, Localization of BrdU label-retaining cells adjacent to a known stem cell marker c-Kit (see inset) after a three-month chase in a mouse uterus, reprinted with permission from Szotek et al. (2007). Panels B and C, Xenotransplantation of human myometrial cells into mouse uteri after side population (SP) flow cytometry. SP cells colocalize with human vimentin (Panel B) but non-SP cells do not (Panel C; reprinted with permission from Ono et al. (2007)). ML, myometrium; myoSP, myometrial side population cells; myoMP, myometrial non-SP cells.
4. Endometrial cancer
4.1. Cancer stem cells defined
Several studies have led researchers to define the characteristics of a cancer stem cell in many tissues (Janzen and Scadden, 2006; Lobo et al., 2007; Scadden, 2006). However, we would be remiss if we did not acknowledge that there is some controversy related to the idea of cancer stem cells (Kelly et al., 2007; Kennedy et al., 2007) and the term ‘cancer stem cell’. Others have proposed the term ‘cancer initiating cell’ (Hill and Perris, 2007) which is analogous to the term tumor-initiating cell that is also commonly used. Regardless, like the stem cell required for normal tissue homeostasis, the cancer stem cell presumably has the capacity for self-renewal and can give rise to malignant daughter cells and progeny of phenotypically different non-tumorigenic cancer cells. Although it is agreed that the cell of origin may or may not be derived from a stem cell, it could as likely be derived from a more differentiated progenitor cell or committed progenitor cell that has regained stem-like qualities and functional characteristics (i.e., self-renewal or drug resistance).
4.2. Clinical perspectives on endometrial cancer
It is estimated that in 2008, more than 40,000 women in the United States alone will be diagnosed with endometrial cancer (Jemal et al., 2008), and it is not difficult to surmise that aberrations in endometrial stem/progenitor cells could lead to a pathologic neoplastic change. Endometrial cancer presents with different histological subtypes that include endometrioid, serous, and mucinous. The histological subtypes are defined by their characteristic resemblance to endometrial (see Figure 5A–D), oviductal (see Figure 5E–F), and cervical tissue (see Figure 5G–H), respectively. The potential to develop into the various subtypes is likely due in part to their Müllerian duct lineage. It is believed that the epithelial/mesenchymal lining of these tissues is less differentiated and therefore has the capacity (under aberrant conditions) to become modified. The diverse phenotypes to which endometrial cancer manifest during the differentiation (or dedifferentiation) process hints at the putative plasticity that these cells potentially possess or have acquired. Therefore it is plausible that a rogue stem/progenitor cell that has been modified, either epigenetically during a differentiation process, or in combination with cumulative genetic mutations resulting from oxidative stress (e.g., aging) or environmental exposure, can serve to bring about the development of individual endometrioid, serous, and/or mucinous cancer subtypes. However, other factors may also influence the development of the different endometrial cancer subtypes including factors influencing the epithelial/mesenchymal transition process and stromal:epithelial interactions, both of which have been implicated in gynecologic disease processes (Auersperg et al., 2001; Auersperg and Woo, 2004; Yoshida et al., 2006). Normally, epithelial stem cells are dependent on these interactions with adjacent stromal cells that comprise specialized niches, which are required for the maintenance of the identity and self-renewal capacity of the stem cell (Clarke et al., 2006; Scadden, 2006). Thus, we hypothesize that stem cells of a gynecologic tumor might evolve differently, generating tumors distinct from their parent cell of origin, by changes to their localized niche environment.
The top two panels represent examples of benign proliferative A and secretory B human endometrium. The second tier provides representative examples of C endometrioid carcinoma imbedded in the myometrium and D clear cell endometrial carcinoma with mucinous differentiation. The third tier displays E uterine papillary serous carcinoma reminiscent of F benign oviductal tissue. The fourth tier demonstrates G mucinous endometrial carcinoma alongside H benign cervical stromal glands. Architectural similarities can be discerned between the endometrioid adenocarcinoma and the proliferative endometrium, the benign oviduct with papillary serous carcinoma and benign cervical glands with mucinous carcinoma.
Current evidence readily supports the notion that only a small fraction of the heterogeneous tumor cells that populate a tumor exhibit stem-like qualities. The remaining cells that make up the bulk of the tumor likely consist of precursor/progenitor cells and the more differentiated cells. It is believed that these more committed cells do not maintain the capacity to either, replicate and form colonies when plated on soft agar, or reconstitute a tumor upon injection and transplantation into immunocompromised mice. The best example of this is an observation that following serial dilution of leukemia cells, where a rare cell phenotype, the leukemia–initiating cell, maintained the capacity to reconstitute a tumor in immunocompromised mice (Bonnet and Dick, 1997). More recent work has provided additional evidence to support the existence of cancer stem cells in solid tumors of the breast, brain, prostate, colon, ovary, and our own work in the endometrium (Al-Hajj et al., 2003; Dontu et al., 2003; Fang et al., 2005; Friel et al., 2008; O’Brien et al., 2007; Patrawala et al., 2006; Singh et al., 2004; Szotek et al., 2006; Zhang et al., 2008). With respect to endometrial cancer we determined that the number of cells required for establishing an endometrial tumor is dramatically reduced in successive serial transplants of primary endometrial carcinomas. Such an approach works well to reduce the ratio of non-tumor initiating cells to tumor initiating cells in vivo. For clarity, it must be noted that the tumors generated in immunocompromised mice following consecutive serial transplants are histologically identical to the original endometrial carcinoma (Friel et al., 2008). Additionally, CM-DiI labeling analyses identified rare label retaining cells within the tumor explants following injection of a labeled endometrial cancer cell line or cells derived from primary endometrial carcinomas.
4.3. Isolation of cancer stem cells
4.3.1. Side population
Enrichment of a unique subpopulation of cells with stem-like properties can be accomplished in some cell types by their inherent ability to exclude Hoechst selectively 33342 dye (Haraguchi et al., 2006; Patrawala et al., 2005; Scharenberg et al., 2002; Szotek et al., 2006) resulting in the isolation of a low and high fluorescing side-population (SP). These are designated SP and non-SP fractions, respectively, by FACS (Preffer et al., 2002). The exclusion process is analogous to the ability of a cell to shuttle toxins out of the cell, a property of some stem cells. The SP fractions are typically sensitive to a calcium channel blocker and potent inhibitor of multi-drug resistance 1 (MDR-1) pump proteins (Goodell et al., 1996; Goodell et al., 1997), verapamil. These rare SP populations have been found in both benign and malignant human endometrium (Friel et al., 2008; Kato et al., 2007). Further, verapamil sensitivity was observed in the SP fractions derived from endometrial cancer cells (Friel et al., 2008). While the SP fraction derived from the malignant tissue represents only a small proportion of the entire tumor cell population, it is postulated to be enriched for stem/progenitor cells.
A more recent study has shown that SP fractions derived from an endometrial cancer line, AN3CA, replicate infrequently, whereas the non-SP fraction turned over relatively rapidly (Friel et al., 2008). Interestingly, the level of the SP fraction was maintained throughout multiple passages and their daughter cells were of non-SP lineage. This idea was confirmed by plating and culturing of labeled endometrial cancer SP cells in clonal assays (Friel et al., 2008). Only the initial clonal cells, at the center of the cellular islands, retained their fluorescence, whereas the surrounding replicating cells did not. Furthermore the SP fraction was more resistant to chemotherapy. This was clearly the case when immunocompromised mice injected with a SP isolated from cultured endometrial cancer cells rapidly developed tumor when compared to mice hosting the non-SP fraction, which had no evidence of tumor (Friel et al., 2008).
4.3.2. Enrichment of cancer stem cells by surface antigens
Isolation of SP cells is only one of several strategies available for the isolation of cancer stem like cells. Cancer stem cells have been identified in breast (Al-Hajj et al., 2003) and brain (Singh et al., 2004) tissues, using markers that identify the stem/progenitor cells in corresponding normal tissues. CD44 is a multi-functional cell surface marker of a host of ‘normal’ stem cells that plays both adhesion and signaling roles (Tang et al., 2007). It has been utilized to isolate stem-like populations from hematopoietic, mesenchymal, breast, and neural cell lineages (Al-Hajj et al., 2003; Avigdor et al., 2004; Oswald et al., 2004; Schwartz et al., 2003). More recently it was used to isolate ovarian cancer-initiating cells from primary tumors (Zhang et al., 2008). Interestingly, CD44 expression is absent in benign proliferative endometrial glands and stroma; whereas only the stroma had evidence of CD44 positive staining in the secretory endometrium (Afify et al., 2006). In contrast, CD44 expression is observed in hyperplastic and malignant endometrial tissue. Moreover the increase in glandular and stromal expression is associated with the progression from hyperplasia, hyperplasia with increased atypia, to adenocarcinoma (Afify et al., 2005). Whether this is a cause or an effect event is not yet known. Furthermore, its role in endometrial stem cell biology has not yet been defined. However, analysis of CD44 expression in a panel of cultured endometrial cancer cell lines revealed highly variable results (Friel et al., 2008). Whether this is attributed to the inability to distinguish between the multiple isoforms or changes observed in response to culture conditions are unclear.
In addition to breast cancer, prostate cancer progenitor cells (Patrawala et al., 2006) have also been isolated using the CD44 high expressing CD24 low expressing phenotype. CD24 is a P-selectin specific cell surface adhesion molecule that is similarly expressed on a variety of epithelial cells (Lim and Oh, 2005), including the ovary (Kristiansen et al., 2002; Santin et al., 2004). Moreover, its expression has been associated with shortened survival period in ovarian cancer (Kristiansen et al., 2002). Cancer stem cells have also been isolated by FACS based on other cell surface markers, such as CD133 (Singh et al., 2004), a marker found on normal primitive stem cells of various lineages (Belicchi et al., 2004; Kim et al., 2002; Yin et al., 1997). CD133 cells have been identified in the benign endometrial epithelium (Schwab et al., 2008); however, there has yet to be any published reports of CD133 positive endometrial tumor/cancer initiating cells.
Although limited, this evidence to support the existence of stem-like cell populations in primary endometrial tumors is relatively convincing. Our findings, in combination with those of others, serve as a basis from which to examine the frequency of the cancer stem/progenitor cell population and its contribution to the development and progression of endometrial cancer. More studies designed to test the functional parameters of these endometrial cancer stem cells are needed for the development of novel strategies for more clinically effective treatment of cancer that targets these cells.
5. Conclusion
It is reasonable to assume that the female reproductive system, and in particular the uterus, owes its tremendous capacity for regeneration to tightly regulated populations of adult stem/progenitor cells that are likely to reside in distinct tissue compartments throughout the female reproductive tract. Although only a handful of articles have been published to date, two types of studies have surfaced over the past four years that offer support for this concept. The first group of studies has relied on non-physiological damage models and bone marrow transplantation. It is clear that bone marrow-derived cells can be found in the uterus; however, it remains to be determined if these cells of extrauterine origin contribute to uterine plasticity or if they simply take on a uterine cell phenotype after being positioned in the right place at the right time as has been demonstrated for hematopoietic stem cells as they integrate into epithelial tumors following intravenous injection (Cogle et al., 2007). Nevertheless, whether or not bone marrow cells contribute to uterine function under physiological conditions seems irrelevant if this phenomenon can be understood mechanistically and exploited for therapeutic purposes. The second group of experiments, completed primarily in vitro, collectively argue that the endometrium harbors cells with substantial replicative and even multipotential ability. Indeed, rare stromal and epithelial cells of the uterus have been identified, grown in culture and, at least in the case of stromal cells, have been induced under defined culture conditions to differentiate into other types of connective tissue. The precise lineage position of these cells, for example if they are true stem cells or merely daughter progenitors, remains in question. Label-retaining and xenograft experiments suggest that myometrial tissue also possesses adult stem cells. These systems now need to be exploited so that the identity of the myometrial stem cell is determined so that the mechanisms of their regulation during homeostasis and its perturbation are better understood. As functional studies are difficult to perform in humans, animal transgenic and xenograft model systems need to be implemented in order to examine the hallmark characteristics of putative uterine stem cells, characteristics shared by all stem cells; that is, the potential for self-renewal and multipotentiality. Once a mechanistic/functional platform has been constructed, it will then be important to determine if and how faulty adult stem cells of the female reproductive tract contribute to gynecologic diseases such as endometrial cancer, fibroids, endometriosis, and pregnancy loss.
Acknowledgments
We are grateful for funding support provided by the Harvard Stem Cell Institute, National Institutes of Health grants HD052701, CA098333, ES012070, the American Society for Reproductive Medicine Organon Research Grant in Reproductive Medicine, Vincent Memorial Research Funds, and the Advanced Medical Research Foundation. We would also like to thank Dr. Chris Crum (Brigham and Women's Hospital, Boston MA) for supplying photomicrographs of oviductal tissue.

