Molecular Motors Direct the Fate of Stem Cells
Groningen, The Netherlands – Scientists at the University of Groningen and the University Medical Center Groningen used molecular motors to manipulate the protein matrix on which bone marrow-derived mesenchymal stem cells are grown. Rotating motors altered the protein structure, which resulted in a bias of the stem cells to differentiate into bone cells (osteoblasts). Without rotation, the stem cells tended to remain multipotent. These results, which could be used in tissue engineering, were published in Science Advances on 29 January.
'Cells are sensitive to the structure of the surface that they attach to', explains Patrick van Rijn, associate professor in Materiobiology and Nanobiomaterials. 'And movement is an important driver in biology, especially continuous movement.' That is why Van Rijn and Feringa and their colleagues decided to use molecular motors to manipulate the protein matrix on which stem cells are grown. The light-driven motor molecules were designed by 2016 Nobel Laureate in Chemistry Ben Feringa.
Structural changes
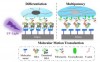
The scientists linked molecular motors to a glass surface. Subsequently, the surface was coated with protein and either exposed to UV irradiation to power the motors or not exposed to it at all. After about an hour, the motor movement was stopped and cells were seeded onto the protein layer and left to attach. Finally, differentiation factors were added. These experiments showed that cells grown on protein that was submitted to the rotary motion of the molecular motors tended to specialize into bone cells more often, while cells seeded on protein that was not disturbed were more inclined to maintain their stem-cell properties.
Observations of the protein layer using atomic force microscopy and simulations of the interaction between the motor molecules and proteins, performed by Prof. Marrink's research group, showed that the movement induced subtle structural changes in the protein matrix. 'The movement of motor molecules interferes with the alpha helices in the proteins, which causes structural changes', explains Van Rijn. He compares it to the difference in texture between an unwhipped egg white and a whipped one.
Cell fate
The change in the surface structure of the adhered protein affects how the cells attach, for example how much they stretch out. This sets off a signalling cascade that eventually leads to altered behaviour, such as the differentiation into bone cells. Thus, molecular movement leads to nanoscopic changes in surface structure, which in turn leads to differences in cell attachment, cell morphology and eventually, cell differentiation. ‘It’s like a domino effect, where smaller stones consecutively topple slightly larger ones so that a large effect can be achieved with a small trigger.'
Changing the properties of a surface to affect cell fate has been used before. However, this was done primarily with switches, so there was just a change from one state to another. 'In our study, we had continuous movement, which is much more in line with the continuous motion found in biological transport and communication systems', says Van Rijn. 'Also, the fact that the motors are driven by light is important Light can be carefully controlled in space and time. This would allow us to create complex geometries in the growth matrix, which then result in different properties for the cells.' Therefore, light-controlled molecular motors could be a useful tool in tissue engineering.
NOTES TO EDITORS
Full Study: Qihui Zhou, Jiawen Chen, Yafei Luan, Petteri A. Vainikka, Sebastian Thallmair, Siewert J. Marrink, Ben L. Feringa, Patrick van Rijn (2020) "Unidirectional rotating molecular motors dynamically interact with adsorbed proteins to direct the fate of mesenchymal stem cells", Science Advance, published online 29 Jan 2020 (DOI: 10.1126/sciadv.aay2756).
Contact
Media contact: Rene Fransen, Rijksuniversiteit Groningen (r.fransen@rug.nl).