1. Introduction
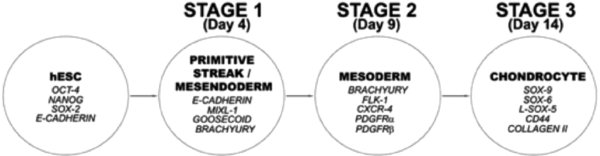
We have developed for hESc a new 3-Stage directed differentiation protocol (DDP) to generate chondrocytes, the specialized cells that form cartilage tissue. The protocol is segmented into stages that mimic the developmental processes that occur in cell lineage specification during embryogenesis. Pluripotent hESc, are established as feeder-free cultures4 prior to DDP being carried out in monolayer culture on a sequence of matrix protein-coated surfaces with a chemicallydefined medium sequentially supplemented with growth factors to directdifferentiation through developmental intermediate cell populations of primitive streak/mesendoderm (Stage 1), mesoderm (Stage 2) and then chondrocytes (Stage 3) (Figure 1). This unique protocol is highly efficient, scalable and completely chemically-defined, thus making it appropriate for translation towards clinical applications of chondrocytes, as well as providing a defined system for characterizing the molecular mechanisms regulating hESc differentiation.
4. Reagents
4.1. Cell culture plastics:
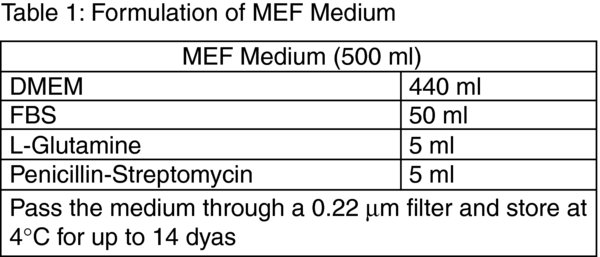
Tip: Due to the decrease in the biological activity of supplementary growth factors we store all culture media at 4ºC and discard 14 days after preparation. In order to prevent waste from discarding expired media we frequently prepare 500 ml volumes of hESc Feeder-Free Medium and Directed Differentiation Basal Medium without the growth factors and store 40 ml aliquots at −20ºC. The required growth factor combinations are then added to thawed aliquots. We have not detected any adverse effects in cell cultures when using media that has been stored at −20ºC for up to 2 months from preparation.
4.2. Growth factors:
Growth factors are purchased as lyophilized powders which are reconstituted to a stock concentration with 0.1% (vol/vol) BSA in DPBS. To prepare 0.1% (vol/vol) BSA add 0.01 g of BSA to 10 ml DPBS, vortex well and sterilize through a 0.22 μm syringe driven filter.
Human recombinant FGF2 (AA10-155) (Biosource™, Invitrogen cat. no. PHG0021) – Dissolve 1 mg of lyophilized powder in 10 ml of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 100 μg/ml (wt/vol). Aliquot the stock growth factor solution in 50 μl volumes.
Human recombinant Activin-A (Peprotech cat. no. 120-14) – dissolve 5 μg of lyophilized powder in 500 μl of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 10 μg/ml (wt/vol). Aliquot the stock growth factor solution in 50 μl volumes.
Human recombinant Neurotrophin-4 (NT4) (Peprotech cat. no. 450-04) – Dissolve 10 μg of lyophilized powder in 1 ml of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 10 μg/ml (wt/vol). Aliquot the stock growth factor solution in 20 μl volumes.
Human recombinant BMP4 (Peprotech cat. no. 120-05) – Dissolve 5 μg of lyophilized powder in 500 μl of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 10 μg/ml (wt/vol). Aliquot the stock growth factor solution in 50 μl volumes.
Human recombinant GDF5 (BMP14/CDMP1) (Peprotech cat. no. 120-01) – Dissolve 50 μg of lyophilized powder in 5 ml of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 10 μg/ml (wt/vol). Aliquot the stock growth factor solution in 50 μl volumes.
Human recombinant Follistatin 300 (Sigma cat. no. F1175-25UG) – Dissolve 25 μg of lyophilized powder in 2.5 ml of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 10 μg/ml (wt/vol). Aliquot the stock growth factor solution in 50 μl volumes.
Mouse recombinant Wnt3a (R&D Systems cat. no. 1324-WN) – Dissolve 2 μg of lyophilized powder is dissolved in 200 μl of 0.1% (vol/vol) BSA in DPBS to make a stock concentration of 10 μg/ml (wt/vol). Aliquot the stock growth factor solution in 25 μl volumes.
Critical: Growth factor polypeptides are highly unstable and biological activity decreases rapidly. Because of this lyophilized growth factors are assumed to be sterile on opening and reconstitution is carried out in a microbiological safety cabinet using aseptic technique. Growth factor solutions are never passed through 0.22 μm filters and cell culture media are sterilized prior to the addition of growth factors. Once reconstituted the growth factor aliquots are transferred on ice for storage at −80ºC. Thawed aliquots are never re-frozen but are stored at 4ºC and discarded 14 days post-thaw.
Cryopreservation medium: Add a 1 ml volume of Dimethyl Sulfoxide (Sigma cat. no. D2650) to 9 ml volume of FBS. Pipette up and down to mix well.
Porcine gelatin (Sigma cat. no. G1890) – A 1% stock gelatin solution is prepared by adding 4 g of lyophilized gelatin powder to 400 ml dH2O. The solution is autoclaved to sterilise, aliquotted in 20 ml volumes and stored at -20ºC. Working gelatin solutions of 0.1% (vol/vol) are prepared in sterile T-75 cell culture flasks by adding 20 ml of thawed 1% (vol/vol) gelatin to 180 ml of sterile dH2O. The 0.1% (vol/vol) gelatin solution is stored at 4ºC and discarded after 28 days
Bovine Serum Albumin (BSA) (Sigma cat. no. A4161) Human Plasma Fibronectin (Millipore cat. no.FC010) – Fibronectin is supplied as a liquid at a concentration of 1 mg/ml (wt/vol) to be stored at 4ºC. Working solutions of 50 μg/ml (wt/vol) are prepared by diluting 1 ml of stock fibronectin in 19 ml of DPBS and stored at 4ºC.
hESc culture on Inactivated Mouse Embryonic Fibroblast (iMEF) Feeder Cell Layer:
-
Transfer 1 ml of 0.1% (vol/vol) gelatin to 1×35 mm culture dish and incubate in a cell culture incubator at 37ºC for 1 hour.
-
Remove one cryovial of iMEF cells from the liquid nitrogen cell storage dewar and place on ice for transfer to the cell culture suite.
-
Hold the cryovial by the lid and submerge three quarters of the cryovial in the 37ºC water bath for 2–3 minutes to thaw the cell suspension. Tip: Swirl the cryovial in the water to facilitate heat transfer. Thaw the iMEF cells until approximately 10% of ice remains, the iMEF cells will continue to thaw during transfer to the microbiological safety cabinet.
-
Draw up 4 ml of MEF medium in a 5 ml serological pipette and then the 1 ml volume of iMEF cell suspension from the cryovial. Transfer the 5 ml volume to a sterile 15 ml polystyrene centrifuge tube.
-
Add 90 μl of the iMEF cell suspension and 10 μl of trypan blue solution to a 1.5 ml microfuge tube and mix well by pipetting up and down. Transfer 10 μl to each chamber of haemocytometer and perform a viable cell count. MEF cells are seeded at a cell density of 3×104 cells/cm2 which for a 35 mm culture dish is 2.92×105 cells; calculate the volume of iMEF cell suspension required: 2.92×105 cells / viable cells/ml = X ml of MEF cell suspension. Transfer the calculated volume to a sterile 15 ml polystyrene centrifuge tube and centrifuge at 720×g for 3 minutes at room temperature. Remove the supernatant and resuspend the cell pellet in 2 ml of MEF medium (Table 1).
-
Take the 35 mm culture dish with gelatin from the incubator, remove the gelatin and add the 2 ml iMEF cell suspension. Transfer to the cell culture incubator.
-
Twenty-four hours post-seeding check the iMEF cells under the microscope to ensure that they have adhered to the cell culture surface and formed a homogeneous culture of approximately 80–85% confluence.
-
Thaw hESc and seed onto the iMEF layer. Remove a vial of hESc from liquid nitrogen storage and thaw as for the iMEF cells in steps 3–5 but substitute hESc medium (Table 2) for MEF medium. Centrifuge the hESc at 720 × g for 3 minutes at room temperature and resuspend the cell pellet in 2 ml of hESc Medium.
-
Remove the iMEF medium from the iMEF cell layer and add the hESc suspension. Transfer the cell culture dish to the cell culture incubator.
-
Change the hESc medium every 2 days and check the hESc for growth under the microscope. Colonies of hESc can be observed growing between iMEF cells after approximately 2–4 days.
-
Passage the hESc at a ratio of 1 : 6 when the culture has grown to approximately 90–95% confluence. Prepare 6 wells of a 6 well culture dish with a iMEF feeder cell layer as described in steps 1–7.
-
Pre-warm a 10 ml aliquot of Trypsin: EDTA and hESc medium by placing in the 37ºC water bath for 30 minutes.
-
Remove and discard the hESc medium from the hESc culture and add 1 ml of Trypsin: EDTA. Incubate in the cell culture incubator at 37ºC and check under the microscope every 2–3 minutes for cell dissociation. iMEF cells are seen to round up whereas hESc can be seen to separate from each other. Critical: Cell:cell contact is crucial in maintaining the hESc phenotype and so it is important that the hESc are not overexposed to the Trypsin: EDTA. The cells should remain in clumps of approximately 5–10 cells to permit the formation of new hESc colonies.
-
Transfer the hESc suspension to a sterile 15 ml polystyrene centrifuge tube and centrifuge at 720 × g for 3 minutes at room temperature. Resuspend the hESc in 12 ml of hESc medium.
-
Remove the MEF medium from the wells of the 6 well culture dish and add 2 ml of the hESc cell suspension to each well. Transfer the culture dish to the cell culture incubator. Tip: We frequently keep 1×6 well cell culture dish of hESc in continuous culture on an iMEF feeder layer. When confluent 1× well is passaged to another 6 well culture dish with iMEFs, 2× wells are taken to establish feeder-free cultures and 3 wells are cryopreserved in order to maintain low passage stocks of hESc.
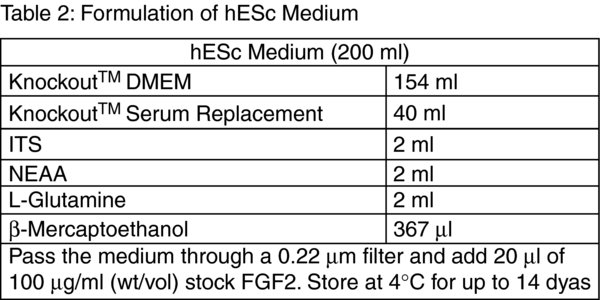
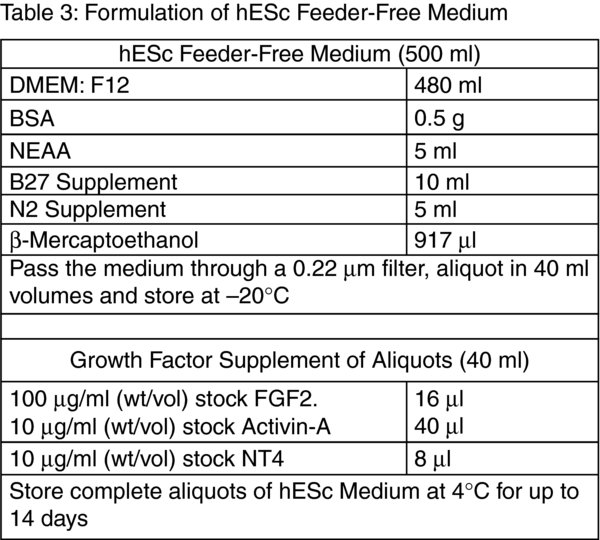
Tip: Prior to assembling the components of the hESc Feeder-Free Medium transfer approximately 20 ml of DMEM: F12 into a sterile 50 ml centrifuge tube containing the 0.5 g of BSA. Vortex the centrifuge tube well to promote complete dissolution of the BSA.
4.3. Feeder-Free hESc Culture:
-
Prepare 35 mm and 6 well culture dishes by transferring 1 ml of 50 μg/ml fibronectin to each well and incubating for at least 16 hours at 4ºC.
-
Remove and discard the hESc medium from 2× wells of hESc iMEF cultures and rinse twice with DPBS.
-
Add 500 μl of Trypsin: EDTA to each of the 2 wells and transfer to the cell culture incubator.
-
Check the hESc cultures every 2–3 minutes as if passaging the hESc cultures on to iMEF feeder cell layers. The Trypsin: EDTA can be left longer to permit more thorough dissociation of the hESc.
-
Transfer the cell suspension to a sterile 15 ml polystyrene centrifuge tube and centrifuge at 720×g for 3 minutes at room temperature.
-
Discard the supernatant and flick the bottom of the tube to disrupt the cell pellet. Resuspend the cells in a 2 ml of hESc Feeder-Free Medium (Table 3).
-
Remove the 50 μg/ml fibronectin from the 35 mm culture dish and transfer to sterile 50 ml centrifuge tube. Tip: The 50 μg/ml working solution of fibronectin can be reused up to three times if it remains at 4ºC.
-
Add the 2 ml volume of hESc cell suspension to the well coated with fibronectin and check under the microscope. Critical: To establish feeder-free cultures hESc should be kept at a high cell density transferring 2× wells of a 6 well culture dish to 1×35 mm culture dish to account for the inclusion of iMEFs. Cell-cell contact is important to maintain hESc phenotype, which is achieved through high cell density. Groups of 2–3 hESc not separated by the Trypsin: EDTA should still be present, however if large numbers of hESc remain aggregated together this will lead to the formation of embryoid bodies and differentiation.
-
Return the 35 mm culture dish to the cell culture incubator.
-
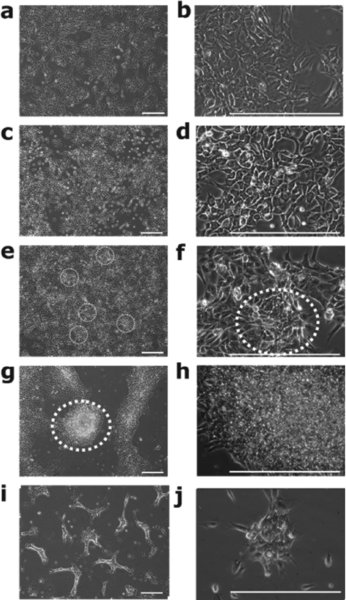
Check the hESc cultures every 24 hours under the microscope for adherence to the fibronectin-coated plastic and replace the hESc Feeder-Free Medium every 24–48 hours. Tip: Feeder-free hESc adhere to the tissue culture plastic as colonies with prominent cell-cell contact. hESc appear larger and flatter than those cultured on iMEF feeder cell layers. Individual cells are seen as having characteristic large nucleus and prominent nucleoli. iMEF cells are often seen between hESc colonies.
-
The feeder-free culture should be passaged at confluence after approximately 3–4 days. Remove the hESc Feeder-Free Medium from the well and add 500 μl of pre-warmed Trypsin: EDTA. Incubate at room temperature and rock the 35 mm culture dish back and forth gently. As soon as the hESc detach add 2 ml of hESc Feeder-Free Medium to the well, draw up the hESc suspension and transfer to a sterile 15 ml polystyrene centrifuge tube. Add another 2 ml of hESc Feeder-Free Medium to the culture dish and rinse out the remaining cells from the well. Pool this with the hESc suspension in the 15 ml tube. Centrifuge the hESc suspension at 720×g for 3 minutes at room temperature and discard the supernatant. Flick the bottom of the centrifuge tube to disrupt the cell pellet and resuspend the hESc in 4 ml volume of hESc Feeder-Free Medium. Transfer 2 ml each to 2× well of a 6 well cell culture dish coated with 50 μg/ml fibronectin as described in step 1. Return the 6 well cell culture dish to the cell culture incubator. Tip: It is important to maintain a high cell density as the hESc culture becomes established, plate cells at a density of approximately 0.8–1×105 cells/cm2. Do not be tempted to passage the feeder-free hESc too early.
-
Repeat steps 10–11 to expand the feeder-free hESc culture. The hESc become adapted to feeder-free culture with increasing passage and as such can be passaged at higher ratios. We routinely passage feeder-free hESc cultures at ratios of 1:3–1:4.
-
To cryopreserve feeder-free hESc cultures detach the cells as if passaging and resuspend the cell pellet in cryopreservation medium. Use 2 ml of cryopreservation medium per 1× confluent well of a 6-well dish (approximately 2×106 cells). Aliquot 1 ml volumes into pre-labelled cryovials, place the cryovials into the “Mr Frosty” and transfer to −80ºC. After 24 hours transfer the cryovials to a liquid nitrogen cell storage dewar.
5. Directed differentiation protocol
The directed differentiation protocol is carried out on feeder-free hESc which have been cultured off feeders for at least 3 passages to exclude contaminating iMEFs.
-
Coat 1×35 mm culture dish by adding 1 ml of 50 μg/ml fibronectin and incubating overnight at 4ºC.
-
Detach feeder-free hESc from the cell culture plastic using pre-warmed Trypsin: EDTA, centrifuge at 720×g for 3 minutes at room temperature and resuspend to a cell density of 5×105 cells/ml in hESc Feeder-Free Medium.
-
Remove the fibronectin solution from the well of the 35 mm culture dish and add a 2 ml volume of feeder-free hESc suspension.
-
Return the culture dish to the cell culture incubator.
-
After 24 hours check the hESc culture under the microscope. DDP should be initiated when the cultures are at approximately 80% confluence. Denote this as Day 1.
-
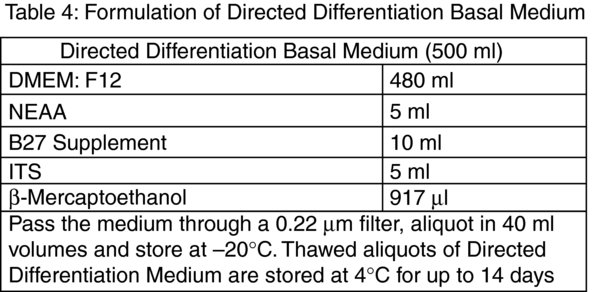
Prepare Day 1 medium by adding 6.25 μl of 10 μg/ml mouse recombinant Wnt3a and 12.5 μl of 10 μg/ml human recombinant Activin-A to 2.5 ml of Directed Differentiation Basal Medium (Table 4).
-
Remove the hESc Feeder-Free Medium, rinse the feeder-free hESc culture three times with DPBS and add 2.5 ml of Day 1 medium. Return to the cell culture incubator.
-
Day 2: add 6.25 μl of 10 μg/ml mouse recombinant Wnt3a; 6.25 μl of 10 μg/ml human recombinant Activin-A and 0.5 μl of 100 μg/ml human recombinant FGF2 to 2.5 ml of Directed Differentiation Basal Medium.
-
Remove the Day 1 medium from the cell culture and replace with 2.5 ml of Day 2 medium. Return to the cell culture incubator.
-
Day 3: add 6.25 μl of 10 μg/ml mouse recombinant Wnt3a; 2.5 μl of 10 μl/ml human recombinant Activin-A; 0.5 μl of 100 μg/ml human recombinant FGF2 and 10 μl of 10 μg/ml human recombinant BMP4 to 2.5 ml of Directed Differentiation Basal Medium.
-
Remove the Day 2 medium from the cell culture and replace with 2.5 ml of Day 3 medium. Return to the cell culture incubator.
-
Day 4: Analyse Stage 1 cultures if appropriate.
-
Add 0.5 μl of 100 μg/ml human recombinant FGF2; 10 μl of 10 μg/ml human recombinant BMP4; 25 μl of 10 μg/ml human recombinant follistatin and 0.5 μl of 10 μg/ml human recombinant NT4 to 2.5 ml of Directed Differentiation Basal Medium.
-
Remove the Day 3 medium from the cell culture and replace with 2.5 ml of Day 4 medium. Return to the cell culture incubator.
-
Transfer 1 ml of 50 μg/ml fibronectin to each of 5 × wells of a 6 well culture dish and place at 4ºC.
-
Day 5: add 2 μl of 100 μg/ml human recombinant FGF2; 40 μl of 10 μg/ml human recombinant BMP4; 100 μl of 10 μg/ml human recombinant follistatin and 2 μl of 10 μg/ml human recombinant NT4 to 10 ml of Directed Differentiation Basal Medium.
-
Remove the Day 4 medium from the cell culture and add 500 μl of pre-warmed Trypsin: EDTA. Incubate at room temperature and rock the 35 mm culture dish back and forth gently. As soon as the cells detach add 2 ml of Directed Differentiation Basal Medium to the well, draw up the cell suspension and transfer to a sterile 15 ml polystyrene centrifuge tube. Add another 2 ml volume of Directed Differentiation Basal Medium to the culture dish and rinse out the remaining cells from the well. Pool this with the cell suspension. Centrifuge the cell suspension at 720×g for 3 minutes at room temperature and discard the supernatant. Flick the bottom of the centrifuge tube to disrupt the cell pellet and resuspend the cells in 10 ml of Day 5 Medium. Remove the 50 μg/ml fibronectin solution and transfer 2 ml of the cell suspension to each of the 5 wells. Return the culture dishes to the cell culture incubator.
-
Day 6: add 2 μl of 100 μg/ml human recombinant FGF2; 40 μl of 10 μg/ml human recombinant BMP4; 100 μl of 10 μg/ml human recombinant follistatin and 2 μl of 10 μg/ml human recombinant NT4 to 10 ml of Directed Differentiation Basal Medium.
-
Remove the Day 5 medium from the 5 cell culture dishes and replace each with 2 ml of Day 6 medium. Return to the cell culture incubator.
-
Day 7: add 2 μl of 100 μg/ml human recombinant FGF2; 40 μl of 10 μg/ml human recombinant BMP4; 100 μl of 10 μg/ml human recombinant follistatin and 2 μl of 10 μg/ml human recombinant NT4 to 10 ml of Directed Differentiation Basal Medium.
-
Remove the Day 6 medium from the 5 cell culture dishes and replace each with 2 ml of Day 7 medium. Return to the cell culture incubator.
-
Transfer 10 ml of 50 μg/ml fibronectin and 10 ml of 0.1% (vol/vol) gelatin to a sterile 50 ml polystyrene centrifuge tube and mix well by pipetting up and down.
-
Transfer 1 ml of the mixed fibronectin:gelatin solution to 20x wells of 6 well culture dishes and place at 4ºC.
-
Day 8: add 8 μl of 100 μg/ml human recombinant FGF2; 160 μl of 10 μg/ml human recombinant BMP4 and 8 μl of 10 μg/ml human recombinant NT4 to 40 ml of Directed Differentiation Basal Medium.
-
Remove the Day 7 medium from the cell cultures and add 500 μl of pre-warmed Trypsin: EDTA to each of the 5 wells. Incubate at room temperature; rocking the cell culture dish back and forth gently. As soon as the cells detach add 2 ml of Directed Differentiation Basal Medium to each cell culture dish, draw up the cell suspension and transfer to a sterile 15 ml polystyrene centrifuge tube. Add another 2 ml of Directed Differentiation Basal Medium to the culture dishes and rinse out the remaining cells from the well. Pool this with the cell suspension. Centrifuge the cell suspension at 720×g for 3 minutes at room temperature and discard the supernatant. Flick the bottom of the centrifuge tube to disrupt the cell pellet and resuspend the cells in 40 ml of Day 8 Medium. Remove the fibronectin:gelatin solution and transfer 2 ml of the cell suspension to each of the 20 wells of the culture dishes. Return the culture dishes to the cell culture incubator.
-
Day 9: Analyse Stage 2 cultures if appropriate.
-
Add 8 μl of 100 μg/ml human recombinant FGF2; 80 μl of 10 μg/ml human recombinant BMP4; 80 μl of 10 μg/ml human recombinant GDF5 and 8 μl of 10 μg/ml human recombinant NT4 to 40 ml of Directed Differentiation Basal Medium.
-
Remove the Day 8 medium from the cell cultures and replace with 2 ml of Day 9 medium to each well. Return to the cell culture incubator.
-
Day 10: add 8 μl of 100 μg/ml human recombinant FGF2; 80 μl of 10 μg/ml human recombinant BMP4; 80 μl of 10 μg/ml human recombinant GDF5 and 8 μl of 10 μg/ml human recombinant NT4 to 40 ml of Directed Differentiation Basal Medium.
-
Remove the Day 9 medium from the cell cultures and replace with 2 ml of Day 10 medium to each of the wells. Return to the cell culture incubator.
-
Day 11: add 8 μl of 100 μg/ml human recombinant FGF2; 160 μl of 10 μg/ml human recombinant GDF5 and 8 μl of 10 μg/ml human recombinant NT4 to 40 ml of Directed Differentiation Basal Medium.
-
Remove the Day 10 medium from the cell cultures and replace with 2 ml of Day 11 medium to each well. Return to the cell culture incubator.
-
Transfer 1 ml of 0.1% (vol/vol) gelatin solution to 30 × wells of 6 well cell culture dishes and place at 4ºC.
-
Day 12: add 12 μl of 100 μg/ml human recombinant FGF2; 240 μl of 10 μg/ml human recombinant GDF5 and 12 μl of 10 μg/ml human recombinant NT4 to 30 ml of Directed Differentiation Basal Medium. Note: This makes a 2 × concentration of Day 12 medium.
-
Remove the Day 11 medium from the cell cultures and add 500 μl of pre-warmed Trypsin: EDTA to each well. Incubate at room temperature; rocking the cell culture dishes back and forth gently. As soon as the cells detach add a 2 ml volume of Directed Differentiation Basal Medium to each cell culture dish, draw up the cell suspension and transfer in equal volumes to 2 × sterile 50 ml polystyrene centrifuge tubes. Add another 2 ml volume of Directed Differentiation Basal Medium to the culture dishes and rinse out the remaining cells from the well. Transfer this cell suspension in equal volumes to the 2×50 ml centrifuge tubes. Centrifuge the cell suspension at 720×g for 3 minutes at room temperature and discard the supernatant. Flick the bottom of the centrifuge tube to disrupt the cell pellet and resuspend the cells in 30 ml of Directed Differentiation Basal Medium. Remove the 0.1% (vol/vol) gelatin solution and transfer 1 ml of the cell suspension to each of the 30 wells of the 6 well culture dishes.
-
Add 1 ml of the 2 × Day 12 medium to each of the 30 wells. Return the culture dishes to the cell culture incubator.
-
Day 13: add 12 μl of 100 μg/ml human recombinant FGF2; 240 μl of 10 μg/ml human recombinant GDF5 and 12 μl of 10 μg/ml human recombinant NT4 to 30 ml of Directed Differentiation Basal Medium. Note: This makes a 2 × concentration of Day 13 medium.
-
Remove the Day 12 medium from the cell cultures and replace with 1 ml each of Directed Differentiation Basal Medium to each of the 30 wells of the 6 well cell culture dishes
-
Add 1 ml each of the 2 × Day 13 medium to each of the 30 wells. Return to the cell culture incubator.
-
Day 14 terminate the cultures and carry out Stage 3 (end point) analyses.
Last revised March 28, 2012. Published June 10, 2012. This chapter should be cited as: Oldershaw Rachel A., Baxter Melissa A., Lowe Emma T., Bates N., Grady Lisa M., Soncin F., Brison Daniel R., Hardingham Timothy E., and Kimber Susan J., Chondrocyte protocol (June 10, 2012), StemBook, ed. The Stem Cell Research Community, StemBook, doi/10.3824/stembook.1.56.1, https://www.stembook.org.